
11 June, 2019
Having recognized the potential of hybrid organic-inorganic perovskites solar cells, in recent years the photovoltaic community has shifted its focus away from efficiency improvements to simplifying the processing and improving the stability of devices.
In this work, we utilize in situ and time-resolved X-ray scattering to track various phase evolutions during the perovskite film solidification to link the microstructure to the composition. In particular, we unravel the crucial roles of Cs+ and Rb+ in promoting the in situ formation of the perovskite phase prior to thermal annealing, thus preventing segregation of halides and cations.
Our study points to a significant new guideline for designing mixed-halide mixed-cation perovskites: the sol-gel formulation must possess the ability to convert directly into the targeted perovskite phase without transitioning through compositionally distinct intermediate phases in order to minimize halide segregation and yield-homogenized films.
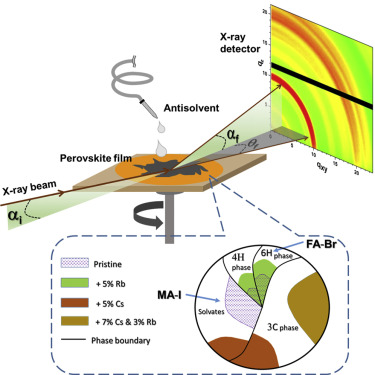
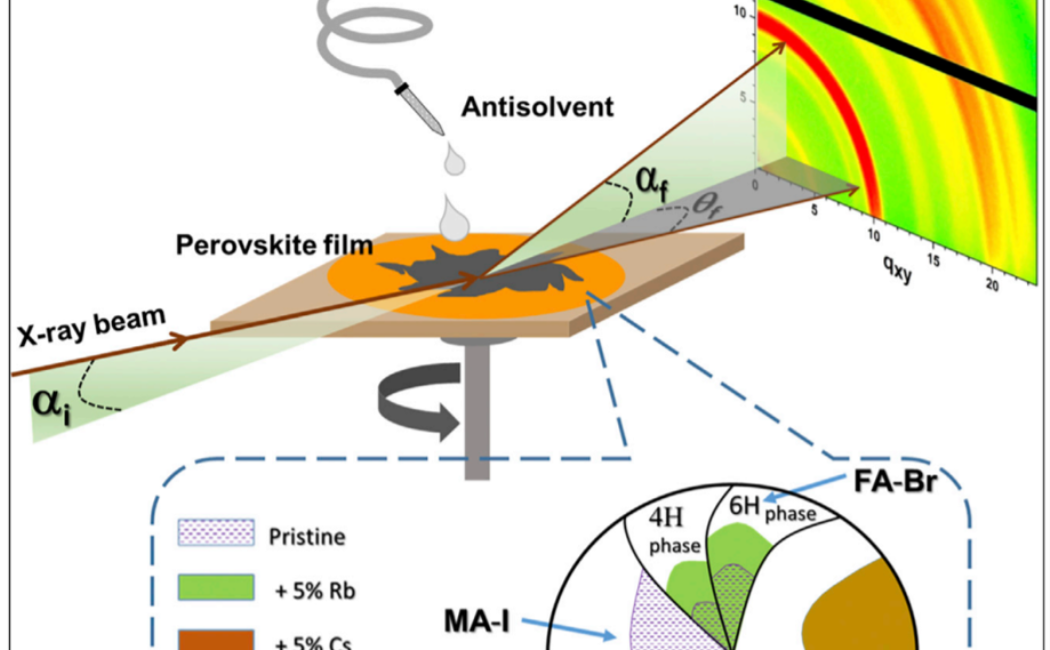
Figure. Inherent phase segregation occurs during the pristine film crystallization, resulting in non-uniform halide and cation compositions, including MA-I-rich ordered solvates and FA-Br-rich 6H phase. Addition of Cs+ and Rb+ jointly conducts the
perovskite orchestra: they synergistically promote the crystallization of a single 3C perovskite phase with excellent optoelectronic properties when used collectively but promote multiple competing phases when used alone or absent, resulting in
halide segregation and deficient solar cell performance. (Reference: Dang, H. et al. 2019 Joule)
To read full article please click